FDA Regulation Means an Even Playing Field
Today the FDA finalized the regulation of labeling and effectiveness of sunscreen. In 2007, they issued a white paper seeking comments from the public regarding what manufacturers can say about the effectiveness of sunscreen and related claims; and they received over 3,000 comments.
Up until today, it seemed they were headed for a grading system with products having 1 to 4 stars. As a manufacturer, it was very tough sitting on the sidelines and waiting - should we go into production or should we wait for final regulation. No one knew when they were concluding their findings but there were enough false alarms that made us think that it was sooner rather than later.
Read more: FDA Regulation of Marketing Claims and Testing
What we did was make sure our lab's testing was reasonable and defensible. We contacted the Skin Cancer Foundation to see what their guidelines where but they were vague as well. We needed a waterproof test but there were no FDA protocols. So we decide to move forward and with two weeks before we were set to go into production...... the regulation is finally released. 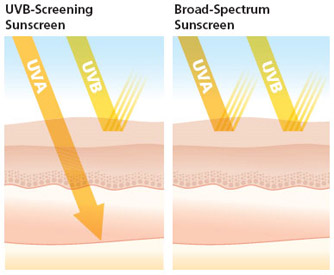
There is nothing but good news here for everyone especially the consumer. Highlights of the regulation include:
1) sunscreens must be tested for protection against both UVA and UVB and if compliant may say "Broad Spectrum";
2) Banning exaggerated claims like Water Proof and Sweat Proof -only Water Resistant will be allowed if the product meets the test;
3) SPF numbers will be capped at 50; and finally
4) Products that are labeled both as Broad Spectrum with SPF values of 15 or higher may state that they reduce the risk of skin cancer and early skin aging.
Any product that is not Broad Spectrum, or that is Broad Spectrum but has an SPF between 2 to 14, will be required to have a warning stating that the product has not been shown to help prevent skin cancer or early skin aging. Changes will take effect next summer.
Read more: The 3 Worst Reasons Not To Wear Sunscreen
The best anti-aging regimen is the daily use of a broad spectrum SPF 30. With this regulation the FDA will now eliminate marketing hype and consumers have a factual baseline in which to judge products.
The final question is how long it will take for anti- aging products without SPF 30 to convert in order to be able to make the claims of reducing early skin aging. My thought is not too long. And it will be a welcome change to the industry.

[…] the designation of Broad Spectrum. I have discussed this earlier and you can read about it here http://abeautyfullthing.com/2011/06/15/fda-regulation-means-an-even-playing-field/ Broad Spectrum means it protects the skin from Ultraviolet A (UVA) rays which ages the skin and […]